Contents
Diagnosis
Numerous tests have been developed to aid the diagnosis of HIV; however, only a few tests have application for routine use. It is important for the primary care physician to be familiar with available diagnostic tests and with guidelines regarding patient populations that are appropriate for HIV screening.
Screening Guidelines
Advertisement
Both the United States Preventative Services Task Force (USPSTF) and CDC recommend routine screening for HIV.16,17 The USPSTF recommends that all individuals aged 15 to 65 years and pregnant women be tested for HIV at least once regardless of perceived risk factors. Younger adolescents and older adults who are at increased risk of infection also should be screened.
Patients who have a high risk for contracting HIV should be screened at more frequent intervals; the CDC recommends at least yearly screening for high-risk patients. Individuals considered to be at an increased risk of infection include men who have sex with men, those engaging in active injection drug use, as well as those who have other sexually transmitted infections or who request testing for STIs. Other behavioral risk factors for HIV infection include having unprotected anal or vaginal intercourse, having HIV-infected sexual partners, or having sexual partners who are bisexual or have a history of intravenous drug use. Receiving money or drugs in exchange for sex is also a high-risk behavior.16
To decrease barriers to HIV testing, the CDC recommends not mandating written consent for HIV testing or counseling. Testing laws vary by state, but most are consistent with these CDC recommentations.18
Diagnostic Tests
To diagnose HIV infection, the CDC recommends beginning with a combination immunoassay that detects HIV-1 and HIV-2 antibodies as well as the HIV-1 p24 antigen in the serum or plasma. If this test is reactive, then a confirmatory test is needed to differentiate HIV-1 from HIV-2 antibodies. If the HIV-1/HIV-2 antibody differentiation assay is intermediate or negative, further testing with a HIV-1 nucleic acid test (NAT) is needed. A reactive NAT confirms the diagnosis of HIV-1 infection.
The current diagnostic test is notably different from the previously used HIV enzyme-linked immunosorbent assay (ELISA) and the confirmatory Western blot test. The new laboratory testing algorithm19 (Figure 1) has advantages over the previous recommended test including improved accuracy of diagnosis of acute HIV-1 infection, more accurate diagnosis of HIV-2 infection, fewer indeterminate test results, and faster turnaround time.
Figure 1: Recommended diagnostic laboratory HIV testing algorithm for serum or plasma specimens
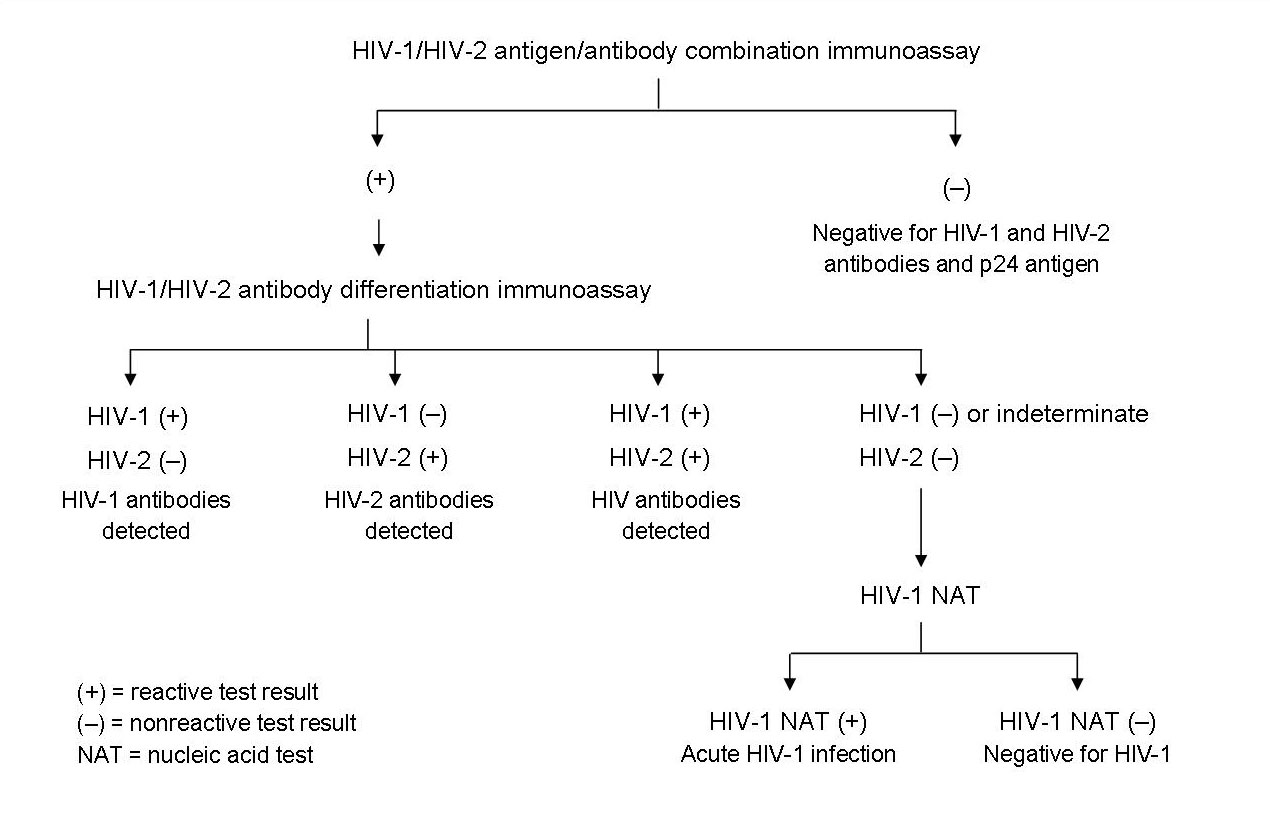
Reprinted from Centers for Disease Control.19
Current CDC recommendations do not include the rapid HIV-1/HIV-2 antigen/antibody combination test that was approved by the Food and Drug Administration (FDA) in August 2013 because of insufficient evidence. The recommendations also do not include non-FDA approved HIV-2 nucleic acid tests. That being said, a positive result on the rapid combination test requires further investigation with the above stated algorithm.19
Evaluation of Patients with HIV
Once a diagnosis of HIV infection has been made, several baseline laboratory studies should be obtained to establish an appropriate treatment plan for the patient. HIV infection should be staged with a CD4+ count and HIV RNA viral load measurement. Genotypic resistance testing is recommended irrespective of whether antiretroviral therapy initiation is deferred. If antiretroviral therapy is deferred, reassessing genotype can be considered. In patients with viral loads below 500 to 1,000 copies per mL, the viral amplification performed for resistance testing may not be successful.20 Additional studies include complete blood count, complete metabolic panel (including transaminase levels), urinalysis, and hepatitis A, B, and C serologies. Fasting lipids and glucose should be checked as well as testing for genotypic resistance.
Patients should also be tested for concomitant Mycobacterium tuberculosis infection via either tuberculin skin test of interferon gamma release assay. A baseline chest x-ray, serologic testing for Toxoplasma gondii, and screening for syphilis are also indicated. Depending on exposure history, gonorrhea and chlamydia screening should be ordered.
HLA B*5701 screening should be ordered if abacavir is part of the treatment regimen. If therapy with a CCR5 antagonist is planned, it is important to order a coreceptor tropism assay.
Women with HIV should have a Pap smear on initial diagnosis. A Pap smear should be repeated every 6 months until results are normal, at which point the screening interval is lengthened to every 12 months. Women with HIV should also be screened for trichomoniasis.21
A complete list of necessary additional testing is provided in Table 3.
Table 3: Initial laboratory evaluation of patients with HIV
|
HIV = human immunodeficiency virus; IgG = immunoglobulin G; PPD = purified protein derivative; RNA = ribonucleic acid; VDRL = venereal disease research laboratory. |
Data from US Department of Health and Human Services.20
< Signs & Symptoms | Treatment >

